Introduction
The oral cavity contains some of the most varied and vast flora in the entire human body and is the main entrance for 2 systems vital to human function and physiology, the gastrointestinal and respiratory systems. Several diseases involve these 2 systems and manifest in the oral cavity. In addition, a specific pathologic condition, such as periodontitis (ie, inflammation of the periodontal attachment of the teeth and the alveolar bone), may be present in the oral cavity. These specific conditions in the oral cavity may create foci of infection that can affect many other vital systems, such as the cardiovascular and renal systems. Foci of infection in the oral cavity arising from chronic periodontitis or chronic periapical abscesses (ie, inflammation and abscess of the tissue attached to the apex of the root) may lead to subacute bacterial endocarditis (BE) and glomerulonephritis (GN).
In addition to bacterial organisms, oral microorganisms can include fungal, protozoal, and viral species. The bacteria include hundreds of types of organisms of which only "22 predominant ones have been identified."1 A variety of organisms in the microenvironment of the oral cavity adhere to the teeth, the gingival sulcus, the tongue, and the buccal mucosa. Each site has a unique way of allowing the organisms to establish their residency. The normal flora in healthy individuals maintains similar patterns. When a local or systemic disease process or concomitant use of medications alters this overall pattern, atypical organisms begin to predominate and some normal organisms with a benign nature, such as Candida albicans, become pathogenic.
The microenvironment of the oral cavity changes with the age of the patient, the eruption or loss of teeth, and the appearance of disease states (eg, caries, periodontal disease). Systemic changes, such as pregnancy or drug intake, also alter the number and proportion of flora. These changes are due to alterations in the flow and composition of salivary fluid and in the levels and activity of defense components (eg, immunoglobulins, cytokines) in the saliva.
Increasing evidence indicates that oral microbiota participate in various systemic diseases. Periodontal disease permits organisms to enter deep systemic tissues, such as the carotid atheroma. An association between periodontal pathogens, such as Porphyromonas gingivalis, and atherosclerosis has been suggested because of the pathogen's possible direct effect on atheroma formation. P gingivalis has also been found in carotid and coronary atheromas. It may also invade and proliferate within heart and coronary artery endothelial cells, and, along with Streptococcus sanguis, it may also induce platelet aggregation associated with thrombus formation. Oral microorganisms may also enter the deeper tissue after trauma or surgery, which contributes to the disease process, particularly when they cause BE.
Periodontitis is a common chronic bacterial infection of the supporting structures of the teeth. The host response to this infection is an important factor in determining the extent and severity of the disease. Systemic conditions may modify the extent of periodontitis principally through their effects on normal immune and inflammatory mechanisms.
Several systemic diseases, such as diabetes mellitus, may increase the prevalence, incidence, or severity of gingivitis and periodontitis. Medications such as phenytoin, nifedipine, and cyclosporin are known to predispose to gingival overgrowth and to increase the severity of plaque formation. Immunosuppressive drug therapy and any disease (eg, HIV infection) resulting in suppression of the normal inflammatory and immune mechanisms can cause or enhance severe periodontal diseases. Smoking, which has an adverse effect on periodontal health, also affects this overall disease condition. Specific diseases, such as histiocytosis, may result in necrotizing ulcerative periodontitis.
The severity and prevalence of periodontitis are increased in persons with diabetes and are worse in persons with poorly controlled diabetes. Periodontitis may exacerbate diabetes by decreasing glycemic control. This effect indicates a degree of synergism and a link between the 2 diseases.
The relative risk of cardiovascular disease is doubled in persons with periodontal disease. Periodontal and cardiovascular disease share many common risk and socioeconomic factors, particularly smoking, which is a powerful risk factor for both diseases. The actual underlying etiology of both diseases is complex, as are the potential mechanisms whereby the diseases may be causally linked. The chronic inflammatory state and microbial burden in persons with periodontal disease may predispose to cardiovascular disease in ways proposed for other infections, such as with Chlamydia pneumoniae.
For excellent patient education resources, visit eMedicine's Teeth and Mouth Center. Also, see eMedicine's patient education articles Gingivitis and Periodontal (Gum) Disease. Also of interest may be the eMedicine articles Viral Infections of the Mouth and Noncandidal Fungal Infections of the Mouth.
Bacterial Endocarditis Secondary to Oral Foci of Infection
Dental manipulation has been proven to cause transient bacteremia. Patients who have congenital or acquired cardiovascular defects are at risk for BE. Before the antibiotic era, these infections were fatal. Today, even with the best medical treatment, this disease has a mortality rate of 10-80%. The lesion of BE is a vegetation that develops on a heart valve. BE is relatively rare, with a worldwide incidence of 10-60 cases per 1 million persons per year.2 However, this rate is low considering the more susceptible group of patients with rheumatic fever and those with a previous history of endocarditis; these patients have a 10-30% risk of developing BE. However, only 200 cases of streptococcal endocarditis following dental and genitourinary tract procedures have been reported in the literature.
Etiology
Infective endocarditis occurs when microorganisms enter the bloodstream and infect damaged endocardium or endothelial tissue. It most commonly involves the heart valves (either native or prosthetic), but it may also occur at the site of a septal defect, on the chordae tendineae, or on the mural endocardium.3 The prototypic lesion is at the site of the infection; the vegetation is a mass of platelets, fibrin, microcolonies of microorganisms, and scant inflammatory cells.4 Endocarditis is classified as acute or subacute, which applies to the features and the progression of infection until diagnosis.
Of BE cases, 8-10% are possibly associated with oral infections in the absence of dental manipulation, and a synergistic effect could exist between the presence of periodontal or periapical disease and dental manipulation favoring BE.5 Brushing teeth, chewing gum, or even eating may provoke bacteremia because of the production of micromovements of the teeth within the sockets.6 Oral bacteremia manifesting after tooth extractions is usually transient, lasting less than 15-30 minutes.7
Epidemiology
In developed countries, the incidence of BE varies from 1-5 cases per 100,000 population per year.8 and has increased in recent years, notably in elderly persons. The cumulative rate of prosthetic valve endocarditis is 1.5-3% one year after valve replacement. This risk is greatest during the first 6 months after replacement.4
The rate of endocarditis occurring in an at-risk patient after receiving dental treatment can vary from 0-1 case in 533.9 population. Numerous studies have demonstrated that bacteremia after dental procedures can cause bleeding in 50-90% of patients.9 Patients with oral bacteremia who do not receive antibiotic treatment can have a mortality rate of 100%. Those treated have a mortality rate of 10-80%; patients with early prosthesis valve endocarditis have the highest mortality rate. Morbidity can include congestive heart failure, renal disease, stroke, and scarred heart valves.
Pathology
The oral cavity, the skin, and the upper respiratory tract are the primary portals for Streptococcus viridans; Staphylococcus species; and Haemophilus aphrophilus, Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans,10 Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae (HACEK) organisms,11 with streptococcal and staphylococcal organisms responsible for more than 80% of cases of BE.
The lesions usually manifest as cardiac, embolic, or general lesions. Cardiac lesions are usually valvular, with vegetative lesions occurring on the line of contact with damaged valve cusps. The mitral valve is usually the most commonly affected, involving 28-45% of patients with BE.4 Endothelial injury causes aberrant flows and allows either direct infection by virulent organisms or the development of an uninfected platelet-fibrin thrombus (nonbacterial thrombotic endocarditis). This thrombus serves as a site of bacterial attachment during transient bacteremia.
Pathogenesis
The microorganisms in the blood adhere to the thrombi, leading to further proliferation, increased platelet fibrin deposition, and vegetative formation. Embolizations of these friable lesions are vegetations that detach and lodge in distal arterioles of every organ system, causing infection and infarction of remote tissues, including the extremities, the spleen, the kidneys, the bowel, or the brain.4
Clinical presentation
Septic emboli can manifest as congestive heart failure with infection-induced valvular damage and splenomegaly and splenic abscesses. Neurologic manifestations, such as delirium, headache, and meningeal irritation, may be caused by mycotic aneurysms. Other generalized lesions can cause myalgias, anorexia, backache, fever, chills, sweats, Osler nodes, Janeway lesions, and splinter hemorrhages with clubbing of the fingers. Persons at greatest risk are patients with prosthetic heart valves, previous infective endocarditis, cyanotic congenital heart disease, aortic valve disease, mitral regurgitation, patent ductus arteriosus, ventricular septal defect, or coarctation of the aorta and those who use intravenous drugs.
Diagnosis
The diagnosis is based on clinical, laboratory, and echocardiographic findings. Transthoracic echocardiography is rapid and noninvasive, and it has excellent specificity (98%) for vegetations.12 However, transthoracic echocardiography may be inadequate as much as 20% of the time because of emphysema or body habitus, and it only has a sensitivity of 65% for detecting vegetations. Transesophageal echocardiography detects vegetations in more than 90% of patients with definite endocarditis,4 and it is the optimal method for the diagnosis of prosthetic valve endocarditis and the detection of a myocardial abscess.3
Outcome
The outcome varies according to the causative organism, time to diagnosis, involvement of the prosthetic valves or the aortic valve, intracardiac complications, and major neurologic complications. The overall survival rate for patients with native valve endocarditis caused by S viridans, HACEK organisms, or enterococci ranges from 85-95%. For Staphylococcus aureus native valve endocarditis, the mortality rate is 55-70% in persons who do not abuse intravenous drugs and is 85-90% in those who do. Prosthetic valve endocarditis beginning within 2 months of valve replacement results in mortality rates of 40-50%.4
Treatment
Because of the high morbidity and mortality rates associated with BE, antibiotic prophylaxis is recommended before selective procedures likely to produce bacteremia and endocarditis, as is maintaining good dental hygiene and aggressively treating local infections.4 The benefits of antibiotic prophylaxis are not established, and, in fact, only 50% of patients with native valve endocarditis know they have a valve lesion predisposing to infection. Organisms not targeted by prophylaxis cause more than 35% of infections.
Dental treatments are the procedures most widely accepted as predisposing to endocarditis; however, they are no more frequent during the 3 months preceding this diagnosis than in uninfected matched controls.4 The administration of antibiotic prophylaxis to at-risk patients who are undergoing dental manipulations is a reasonably well-accepted clinical practice.13 The most frequently used guidelines are those of the American Heart Association (see Endocarditis Prophylaxis Information) and the British Society of Antimicrobial Chemotherapy.
Numerous studies have demonstrated that antibiotics can reduce the prevalence and the magnitude of bacteremia.14 Patients who are susceptible may be able to reduce the risk of endocarditis by maintaining good dental hygiene and aggressively treating local infections. The antibiotic regimen for prophylaxis of endocarditis in adults at moderate or high risk is as follows:
- Oral cavity, respiratory tract, esophageal, or minor surgical procedures
- Standard regimen (low-to-moderate risk) - Amoxicillin at 2 g orally 1 hour before the procedure
- Inability to take oral medication - Ampicillin at 2 g intravenously or intramuscularly within 30 minutes of the procedure
- Penicillin allergy
- Clarithromycin at 500 mg orally 1 hour before the procedure
- Cephalexin or cefadroxil at 2 g orally 1 hour before the procedure
- Clindamycin at 600 mg orally 1 hour before the procedure
- Inability to take oral medication
- Cefazolin at 1 g intravenously or intramuscularly 30 minutes before the procedure
- Clindamycin at 600 mg intravenously 30 minutes before the procedure
- Patients at high risk
- Ampicillin at 2 g intravenously or intramuscularly plus gentamicin at 1.5 mg/kg intravenously or intramuscularly (not to exceed 120 mg) within 30 minutes of the procedure; repeat ampicillin at 1 g intravenously or intramuscularly or amoxicillin at 1 g orally 6 hours later
- Penicillin allergy - Vancomycin at 1 g intravenously over 1-2 hours plus gentamicin at 1.5 mg/kg intravenously or intramuscularly within 30 minutes before the procedure, no second dose used
The Medscape CME course New Update on Infective Endocarditis Prophylaxis: Now a Class IIa Recommendation may be of interest.
Cardiovascular and Cerebrovascular Disease Secondary to Oral Foci of Infection
Periodontitis has been shown to be associated with atherosclerosis and coronary heart disease. Periodontal disease is common in persons with these conditions, and it may account for a significant portion of infection-associated risk of cardiovascular disease.15
Etiology
Oral infections may directly contribute to atherogenesis and thromboembolic events by providing repeated systemic vascular challenges of proinflammatory cytokines, periodontal pathogens, and lipopolysaccharide (LPS). The proinflammatory cytokines may be more closely associated with the more chronic aspects of cardiovascular disease, such as the formation and the progression of arterial plaques, whereas the microbial and the LPS exposures may be associated with the more acute aspects of coronary heart disease and stroke, such as thrombus formation.
As with chronic infections elsewhere in the human body, chronic periodontal infections are associated with systemic changes to the blood and blood-forming organs, such as increases in plasma fibrinogen and white blood cells. Other chronic infections, such as Helicobacter pylori gastritis and C pneumoniae pneumonitis, have also been associated with an increased risk of ischemic heart disease. One can postulate that changes to the blood and blood-forming organs (eg, hyperfibrinogenemia) and chronic inflammatory conditions may be mechanisms through which infections promote an increased risk of ischemic diseases. Microorganisms can infect vascular endothelial cells directly, initiating the inflammatory response needed for the initial process of inducing atherosclerosis.16
Epidemiology
Acute episodes of an infectious disease may play a role in precipitating an acute cardiovascular event.16 The infection with the largest body of evidence to support an association with cardiovascular disease and cerebrovascular disease is infection with C pneumoniae. Most of the 30 retrospective and cross-sectional studies show an association between C pneumoniae antibodies and cardiovascular disease. Sufficient evidence from biological mechanisms and animal models warrant interventional studies on periodontitis and the development of cardiovascular events.
A series of case-control and cross-sectional studies has shown a significant association between various indexes of poor dental health and coronary heart disease.15 The odds of having a history of heart attack increase with the severity of periodontal disease.17 The association between specific subgingival periodontal organisms and myocardial infarction (MI) has been evaluated, and 2 periodontopathic organisms, Bacteroides forsythus and P gingivalis, are associated with MI.18 Another study reported that 921 men free of cardiovascular for 18 years had high levels of alveolar bone loss at baseline (measure of periodontal disease) and noted that this was a significant predictor of total cardiovascular disease and stroke.19
Pathology and pathogenesis
The disease mechanism for atheroma formation and thrombosis has been linked to platelet aggregation. The oral gram-positive bacteria, S sanguis, and the gram-negative periodontal pathogen, P gingivalis, found in dental plaque, enter the bloodstream during bacteremic episodes. These bacteria may induce platelet activation and aggregation through the expression of collagenlike platelet aggregation–associated proteins, playing a role in the formation of atheroma and thrombosis.20
Studies suggest that periodontal pathogens may be present in arteriosclerotic plaques and, similar to other infectious organisms (eg, C pneumoniae), may play a role in the development and progression of atherosclerosis. The infection with the largest body of evidence supporting an association with cardiovascular and cerebrovascular association is infection with C pneumoniae. An acute episode of infectious disease may play a role in precipitating an acute cardiovascular event via an acute thrombus dislodging a vulnerable plaque.16
Several studies have found P gingivalis capable of invading the coronary and carotid endothelium in cell cultures.21 Animal studies have suggested that infection with P gingivalis activates the acute-phase response, increasing lipemia and atheroma formation.22 C pneumoniae has proven, in vitro, capable of infecting vascular endothelial cells, smooth muscle cells, and macrophages of human origin.23
Periodontal infections may stimulate the release of cytokines, such as tumor necrosis factor-alpha, interleukin (IL)–1, IL-6, and IL-8. One of these potential stimuli, the endotoxin LPS, is present in the subgingival plaque of patients with severe periodontal disease. LPS and other bacterial components can activate an impressive cascade of inflammatory cytokines, which, in turn, can play a role in atherosclerotic heart disease either through direct action on the vessel wall or by inducing the liver to produce acute-phase proteins.24
C pneumoniae and its LPS can enhance low-density lipoprotein accumulation in human-derived monocytes to form foam cells, which are a key component of atherosclerosis. Microorganisms can infect vascular endothelial cells directly, initiating the inflammatory response needed for the initial process of inducing atherosclerosis and for accelerating or enhancing this process.
Acute-phase proteins, such as C-reactive protein (CRP) and fibrinogen, affect coagulation, platelet activation, and aggregation, contributing to atheroma formation. The LPS and inflammatory cytokines present in periodontal pathogens may also increase the expression of leukocyte adhesion molecules, such as intercellular adhesion molecules or vascular cell adhesion molecules, by endothelial cells. Intercellular adhesion molecules and vascular cell adhesion molecules are associated with atheroma formation.25 Studies have also provided evidence that periodontal disease is associated with cardiovascular risk factors, including CRP and plasma fibrinogen levels, which are systemically increased in people with periodontitis. One study demonstrated that CRP levels are highest in patients infected with periodontal pathogens.26
CRP is also an independent risk factor for cardiovascular disease. CRP localizes with complement in human hearts during MIs, suggesting that CRP binds to diseased muscle tissue, fixes complement, and triggers complement-mediated inflammation that contributes to atheroma formation.27 Periodontal infections may be associated with an increased risk of atherosclerotic processes, such as coronary artery disease and strokes, because of the association of periodontal infections with elevated levels of CRP.
The accumulation of epidemiologic, in vitro, and animal evidence suggests more than just a casual role of periodontal infections as a risk factor for cardiovascular disease and stroke. People with severe periodontitis have the strongest link to cardiovascular disease and cerebrovascular disease. These people also have the greatest amounts of pathogenic bacteria and systemic factors that may contribute to an altered immunoinflammatory response.
Treatment
According to the discussion of the etiology and pathogenesis of atheroma in patients with severe periodontal disease, the goals of treatment are to treat hypercholesterolemia and lower low-density lipoprotein levels via any of the new statin medications in conjunction with a low-fat, low-cholesterol diet. Maintaining good oral hygiene and eliminating the oral foci of infection and bacterial diseases are additional methods used to eliminate the oral cause of atheroma formation.
Glomerulonephritis Secondary to Oral Foci of Infection
In patients with poor oral hygiene and in the presence of oral foci of infection, renal involvement is characterized by the abrupt onset of hematuria and proteinuria and is often association with edema, hypertension, and mild-to-moderate renal insufficiency. The disease usually develops several days after Streptococcus mitis or Streptococcus mutans bacteremia. However, several reported cases indicate that renal disease is not caused by streptococci but is associated with a bacteremic state or with viral or parasitic diseases.
GN is the most common primary renal disease in developing countries; however, it is becoming uncommon in the Western world. This suggests that the development of GN is associated with low socioeconomic status and poor hygienic conditions. This suggestion is bolstered by the observation that cases occur in clusters and epidemics, which, at times, may even be cyclic. GN can affect persons of any age; however, most patients are aged 2-12 years. Proliferative GN is twice as common in males as in females.
Pathology
In the early phases of bacterial infection, diffuse glomerular, mesangial, and endothelial cell proliferation is present and is associated with infiltration of the capillary lumens by polymorphonuclear leukocytes and mononucleotide cells. Extracapillary proliferation may involve a few glomeruli, whereas diffuse and extensive circumferential crescent formation is uncommon, although possible. Inflammatory cells expressing IL-8 and transforming growth factor-beta (TGF-beta) appear to increase the expression of intercellular adhesion molecules, which can be found in both glomerulus and interstitial sites. IL-8 levels are correlated with glomerular neutrophil infiltration, whereas TGF-beta levels are correlated with mesangial matrix proliferation.28 The tubulointerstitial compartment is usually normal, but, in most proliferative forms, acute cellular infiltrates can be seen.
The typical immunohistologic pattern is characterized by abundant and well-defined granular deposits of C3 along the outer aspects of the glomerular capillary walls. These deposits confer the appearance known as starry sky. Immunoglobulin G and immunoglobulin M are present in 60-70% of cases. C1q and C4 are generally lacking. Fibrinogen is seen only in crescents. With electron microscopy, coarse electron-dense subepithelial deposits are the distinguishing feature of GN. Because of their shape, the deposits are defined as humps. However, intramembranous and subendothelial deposits and mesangial deposits can also be found.
In later phases of the disease, endocapillary proliferation and polymorphonuclear infiltration are less evident and prominent mesangial deposits can be observed. In other cases, subepithelial deposits are confluent, so that apparently elongated deposits, which confer a flarelike pattern immunohistologically, replace the humps. In cases without complete resolution, the typical endocapillary disease can transform over time into a mesangiocapillary pattern.
Pathogenesis
Some data suggest that GN is an immune-complex disease in which some components of nephritogenic streptococci likely act as antigens. Cell membrane antigens, streptococcal cationic proteinase, extracellular plasmin-binding protein, and endostreptosin or preabsorbing antigen have been proposed as possible triggers for the formation of the immune complexes. On the other hand, antiimmunoglobulin antibodies could be involved in the pathogenesis of GN, as demonstrated by the high titers of rheumatoid factor and by the presence of antiimmunoglobulin deposits in renal biopsy specimens of some patients.
An important role in conferring antigenicity to immunoglobulins can be played by another streptococcal component, the neuraminidase. In fact, this component can cause consequent modifications of autologous immunoglobulins; moreover, neuraminidase can alter leukocytes and favor their deposition in the glomeruli and the interstitium.
Cellular immunity may also be involved in the pathogenesis of GN, as suggested by glomerular and interstitial infiltration of granulocytes, monocyte/macrophages, and T lymphocytes; increased IL-8 and TGF-beta levels in the kidneys; and increased IL-6 and tumor necrosis factor-alpha levels in the circulation.
Clinical presentation
The typical patient with GN presents with an acute nephritic syndrome (ie, oliguria, edema, hypertension, gross hematuria) 10-21 days after the onset of bacteremia. Nonspecific symptoms, such as malaise, weakness, and nausea, are frequent. Dull lumbar pain is present in 5-10% of patients.29 Although rare, an extrarenal disease is possible in patients with GN, as demonstrated by the association with oliguria, scleritis or cerebral palsy in elderly patients, and vasculitis.
Decreased glomerular filtration and salt and water retention are the main causes of edema and hypertension, as documented by the finding of increased plasma volume and cardiac output in most patients with GN. In children, edema is usually generalized, while in adolescents and adults, it is more frequently confined to the face and the legs. Hypertension is observed in 80% of patients, but only 50% of patients require treatment. However, in rare cases, hypertension may be severe and cause hypertensive encephalopathy.30 Especially in elderly patients, oliguria and fluid retention can cause heart failure and death. Proteinuria is common in persons with GN; however, nephrotic proteinuria is rare, especially in children.31
In other patients, GN can be asymptomatic and cause only a transient serum complement decrease and/or mild urinary abnormalities, such as isolated microscopic hematuria, with or without hypertension.32 Prospective studies in families have shown that this presentation is more common than the overt clinical presentation described above. Very rarely, GN is associated with urinary abnormalities in spite of clinical symptoms and the presence of endocapillary GN at biopsy.
Outcome
Usually, the nephritic symptoms spontaneously reverse 4-7 days after the onset; however, urinary changes, mild renal function impairment, or hypertension can persist or develop months or years after the acute episode. These abnormalities can be found in approximately 20% of patients 1-2 decades after the onset. Usually, children do better than adults. Patients with a nephrotic syndrome at presentation and those with extensive deposits in the peripheral capillary loops have a worse prognosis. Patients with crescentic disease may recover spontaneously. However, the prognosis is poor when crescents involve 60% of glomeruli.33 In some patients with a subclinical presentation, persistent or intermittent microscopic hematuria may be seen at long-term follow-up examinations.
Diagnosis
GN results in increased antistreptolysin titers and decreased C3 levels. Increased antistreptolysin titers are observed in 40-90% of patients with GN. With GN, more than 90% of patients have a decrease in C3 levels and normal C4 serum levels, indicating an activation of the alternative pathway of complement. In most cases, C3 levels return to normal in less than 8 weeks, even though a prolonged decrease of C3 levels is possible. The behavior of complement distinguishes GN from other renal diseases, such as lupus nephritis, in which both C3 and C4 levels are usually reduced in active phases. In type 2 cryoglobulinemic nephritis, usually only the C4 level is strongly decreased.
Usually, no extrarenal symptoms are present in persons with GN besides edema and hypertension. These features differentiate GN from systemic diseases that cause acute nephritic syndrome. However, keep in mind that in occasional patients with GN, extrarenal symptoms (eg, scleritis, cerebral vasculitis) may be present.
GN can be easily diagnosed in epidemic or family cases, even when only transient and minor clinical and urinary changes are present. However, for sporadic cases with only persistent urinary abnormalities, distinguishing between immunoglobulin A nephropathy, thin basement membrane disease, and other conditions can be difficult without performing a renal biopsy. However, a typical history and clinical picture in most patients with acute nephritic syndrome caused by GN make diagnosis easy; thus, a confirmatory biopsy is not needed. Renal biopsy may be indicated for patients with an atypical history or presentation and especially for patients with severe or prolonged renal failure, which can be caused by crescentic disease.
Treatment
The primary goals of treatment are to eliminate oral foci of infection and to manage oliguria, edema, and hypertension. Renal management includes restriction of sodium and fluid intake. Furosemide or other loop diuretics are frequently needed. In many patients, hypertension reverses with the correction of fluid overload and edema. However, in some patients, antihypertensive agents are needed.
Antistreptococcal antibiotics, such as penicillin, cephalosporins, erythromycin, or derivatives, should be given. Corticosteroids or immunosuppressive agents are not indicated in persons with GN because the disease spontaneous resolves in most cases. However, in patients with extensive crescent formation and slow resolution of symptoms, a short course of high-dose glucocorticoids may be considered.
Conclusion
Maintaining good long-term oral hygiene is required in patients with GN and in patients with BE and atherosclerotic cardiovascular disease. Elimination of oral foci of infection as potential sources of bacterial seeding is essential for persons with any of the conditions discussed.
Multimedia
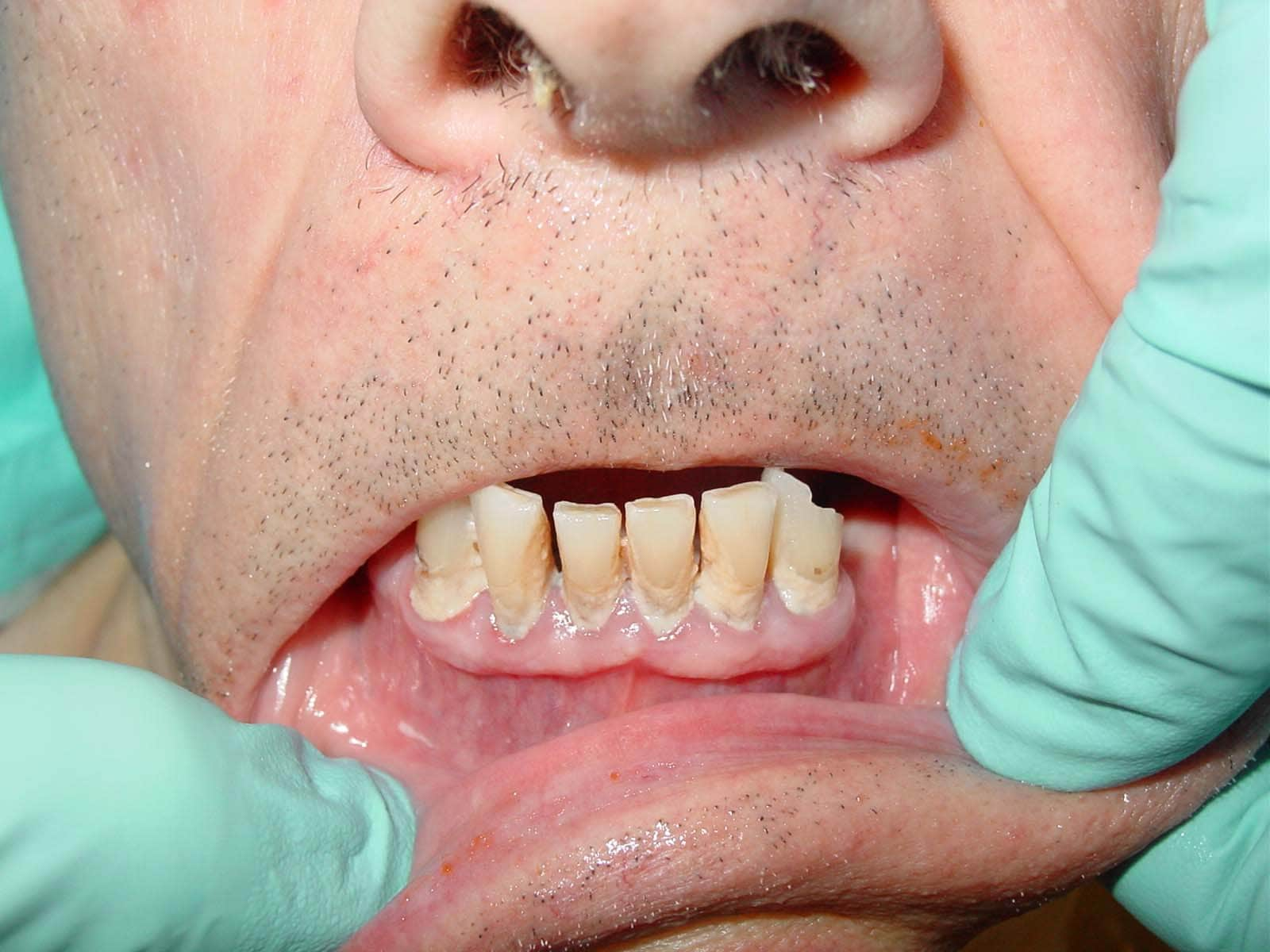
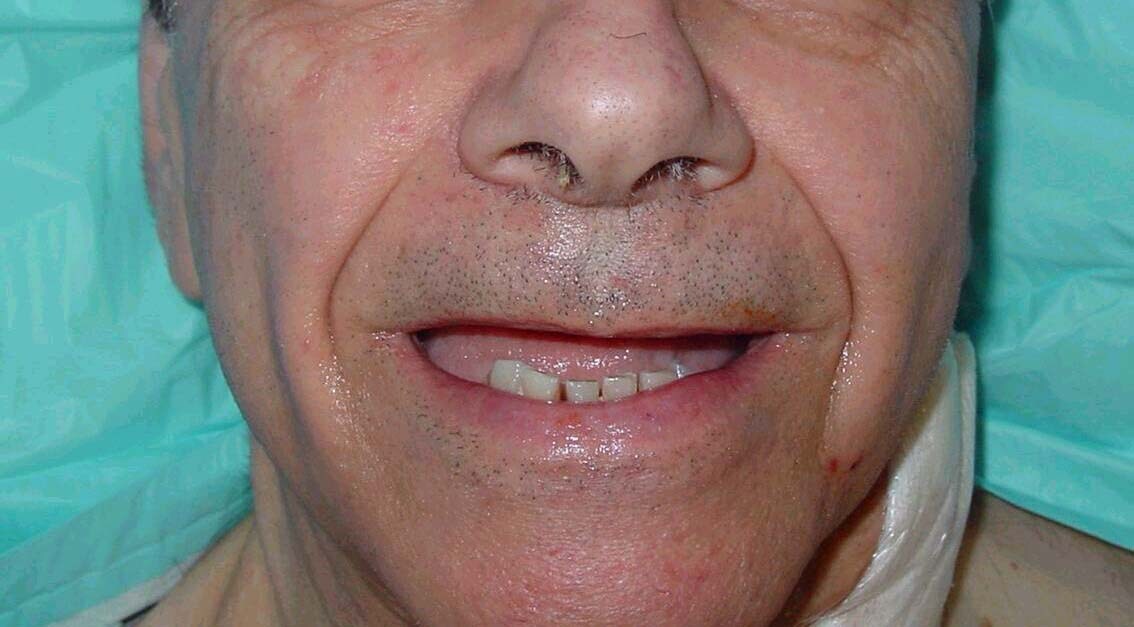
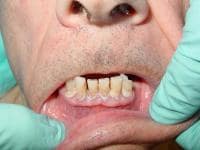 | Media file 1: A 72-year-old man with severe periodontal disease (same patient as in Media Files 2-6). |
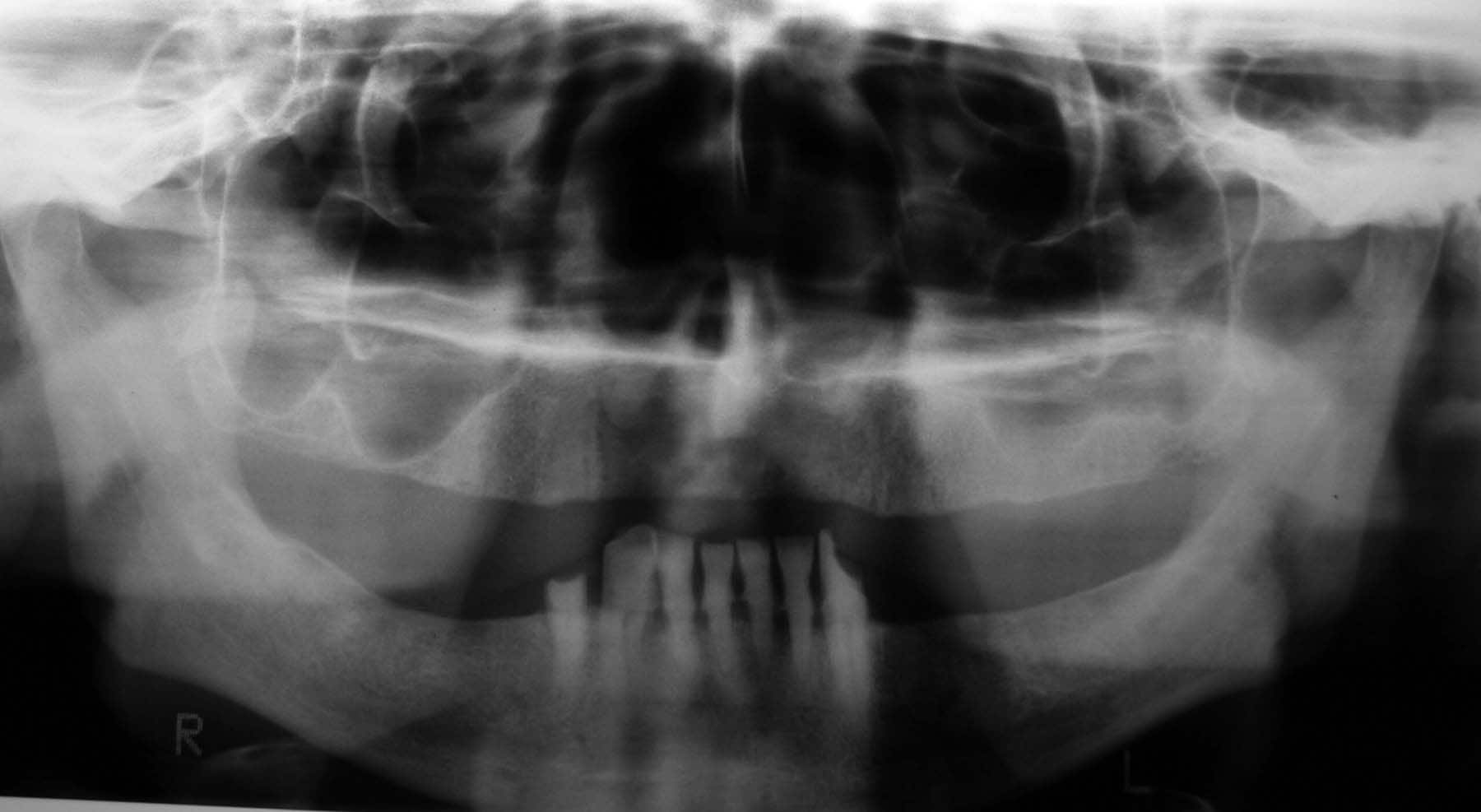
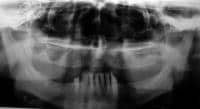 | Media file 2: Radiographically evident severe alveolar bone loss and periodontitis (same patient as in Media Files 1 and 3-6). |
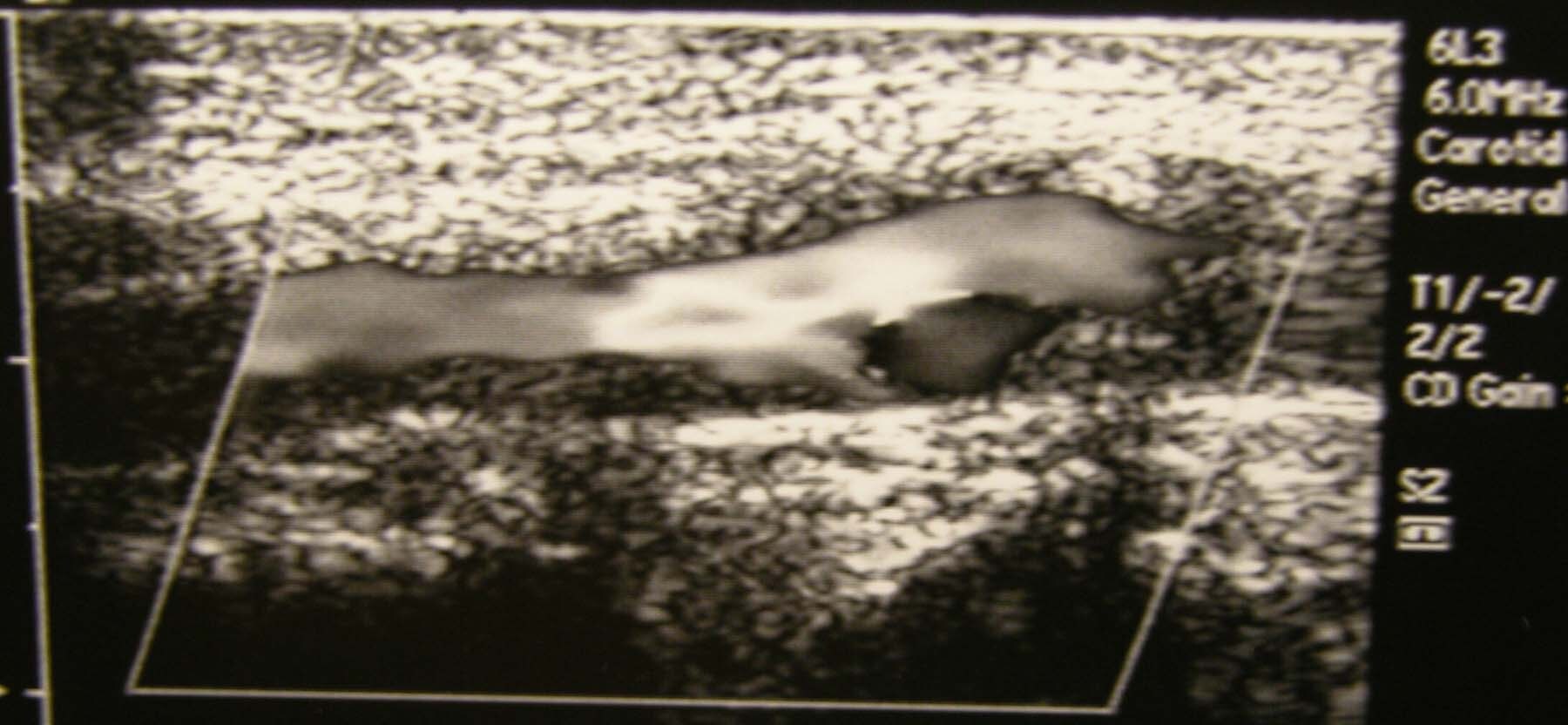
 | Media file 3: Carotid duplex ultrasonogram exhibits severe atherosclerosis/stenosis of the left carotid artery (same patient as in Media Files 1-2 and 4-6). |
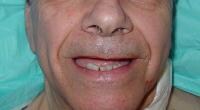 | Media file 4: Status post left carotid endarterectomy (same patient as in Media Files 1-3 and 5-6). |
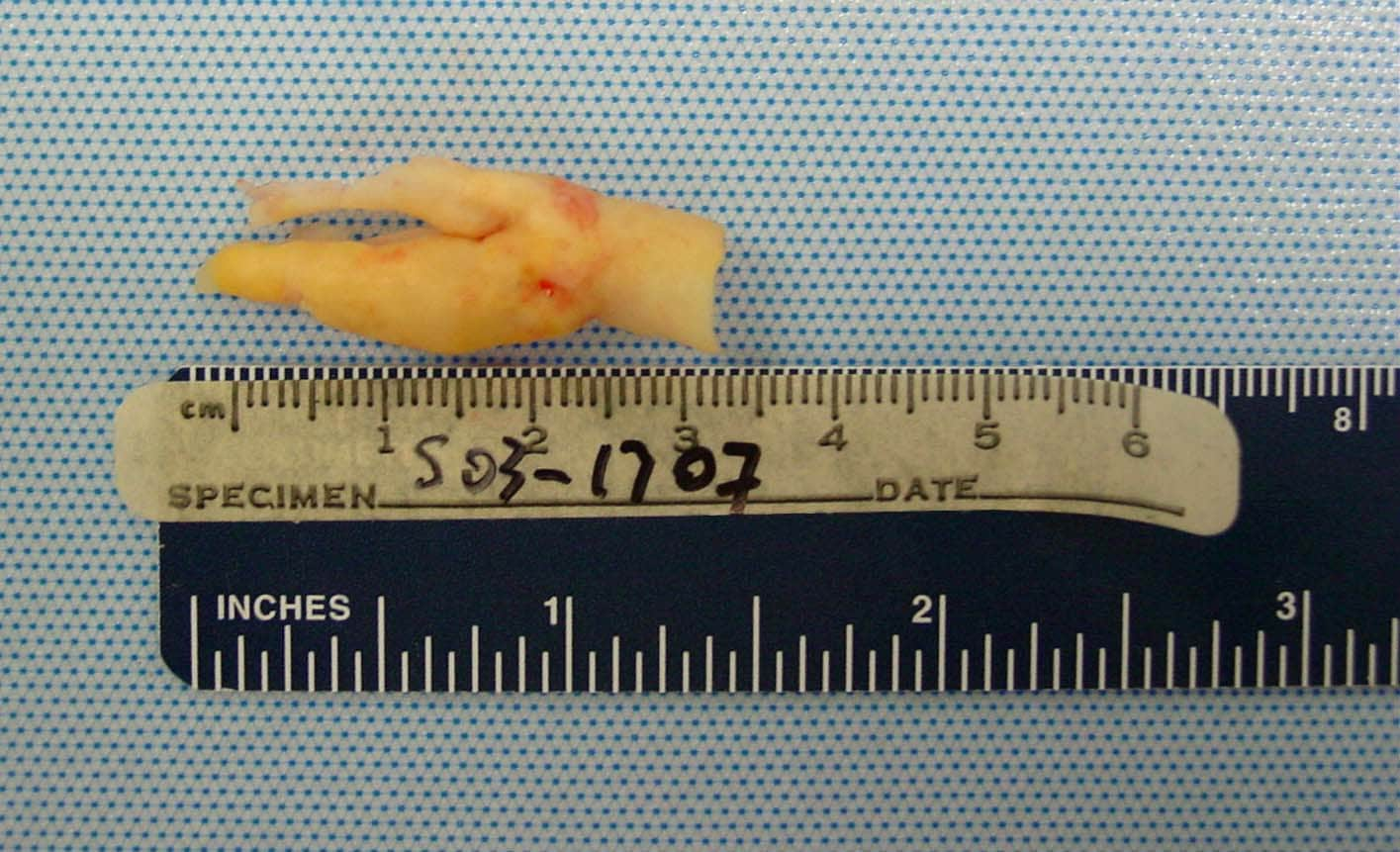
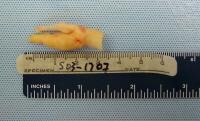 | Media file 5: Gross pathologic specimen shows carotid plaque as cause of stenosis (same patient as in Media Files 1-4 and 6). |
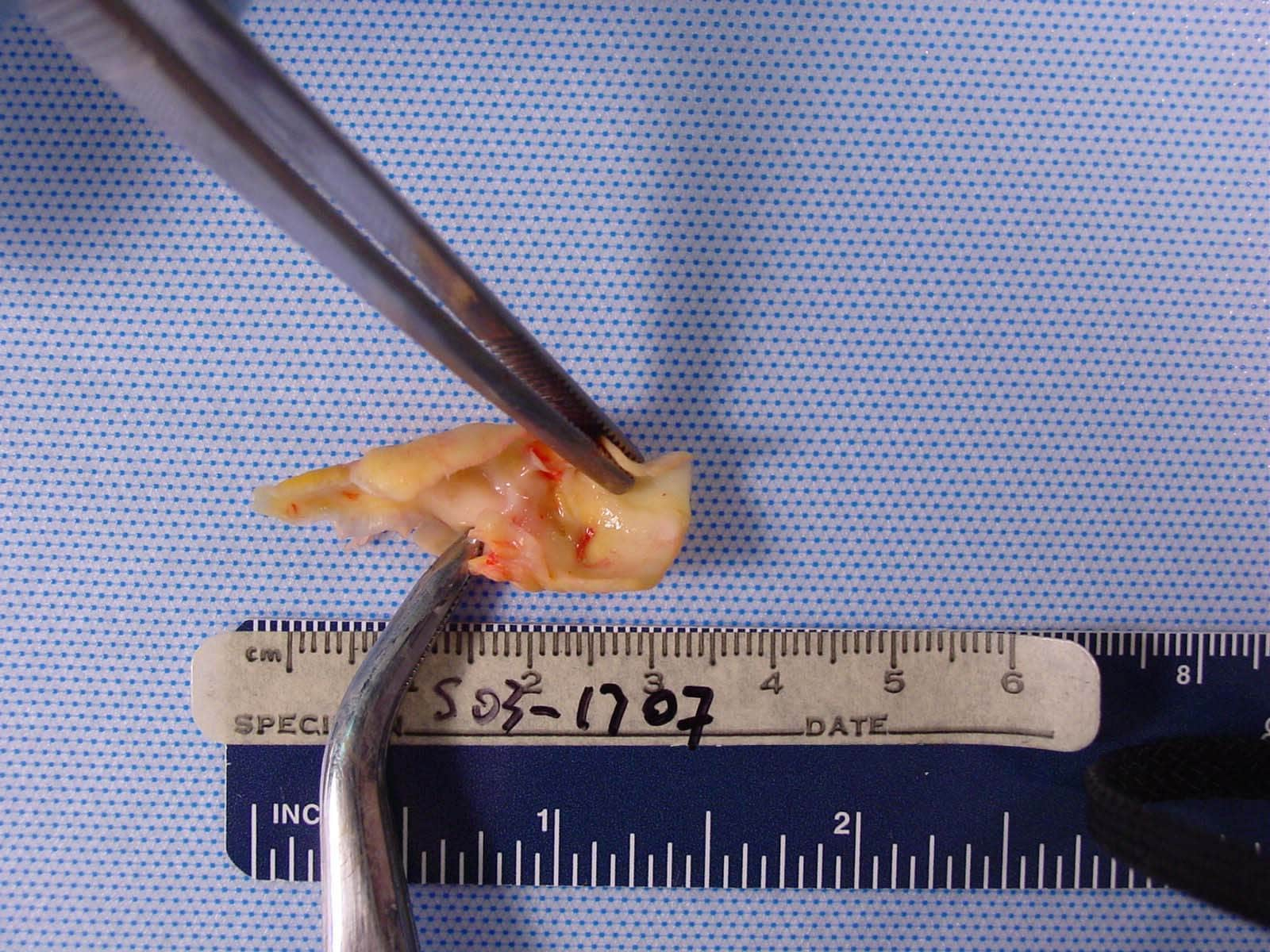
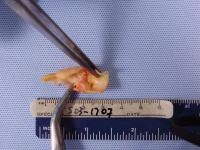 | Media file 6: Dissected pathologic carotid plaque with bacterial contents similar to periodontal plaque surrounding existing dentition (same patient as in Media Files 1-5). |
Keywords
bacterial mouth infections, mouth infection, gingivitis, periodontitis, bacterial endocarditis, BE, subacute bacterial endocarditis, subacute BE, oral foci of infection, cardiovascular disease, cerebrovascular disease, glomerulonephritis, GN, renal disease, bacterial infection of the oral cavity, periodontal disease, Porphyromonas gingivalis, P gingivalis, Streptococcus sanguis, S sanguis, Streptococcus mitis, S mitis, Streptococcus mutans, S mutans, Chlamydia pneumoniae, C pneumoniae, mouth infection, oral infection, mouth disease, mouth lesion, oral lesion, oral ulcers, oral ulceration, mouth ulcers, mouth bacteremia, oral bacteremia, periapical abscess, atheroma formation, atheroma
My life is beautiful thanks to you, Mein Helfer. Lord Jesus in my life as a candle light in the darkness. You showed me the meaning of faith with your words. I know that even when I cried all day thinking about how to recover, you were not sleeping, you were dear to me. I contacted the herbal center Dr Itua, who lived in West Africa. A friend of mine here in Hamburg is also from Africa. She told me about African herbs but I was nervous. I am very afraid when it comes to Africa because I heard many terrible things about them because of my Christianity. god for direction, take a bold step and get in touch with him in the email and then move to WhatsApp, he asked me if I can come for treatment or I want a delivery, I told him I wanted to know him I buy ticket in 2 ways to Africa To meet Dr. Itua, I went there and I was speechless from the people I saw there. Patent, sick people. Itua is a god sent to the world, I told my pastor about what I am doing, Pastor Bill Scheer. We have a real battle beautifully with Spirit and Flesh. Adoration that same night. He prayed for me and asked me to lead. I spent 2 weeks and 2 days in Africa at Dr Itua Herbal Home. After the treatment, he asked me to meet his nurse for the HIV test when I did it. It was negative, I asked my friend to take me to another nearby hospital when I arrived, it was negative. I was overwhite with the result, but happy inside of me. We went with Dr. Itua, I thank him but I explain that I do not have enough to show him my appreciation, that he understands my situation, but I promise that he will testify about his good work. Thank God for my dear friend, Emma, I know I could be reading this now, I want to thank you. And many thanks to Dr. Itua Herbal Center. He gave me his calendar that I put on my wall in my house. Dr. Itua can also cure the following diseases ... Cancer, HIV, Herpes, Hepatitis B, Inflammatory Liver, Diabetis, Bladder Cancer,Colorectal Cancer,Breast Cancer,Kidney Cancer,Leukemia,Lun,Fribroid,Parkinson's disease,Inflammatory bowel disease ,Fibromyalgia, recover your ex. You can contact him by email or whatsapp, @ .. drituaherbalcenter@gmail.com, phone number .. + 2348149277967 .. He is a good doctor, talk to him kindly. I'm sure he will also listen to you.
Trả lờiXóaHere is my Email contact...piresmis59@gmail.com.