History
Flappe is a term from the 16th century denoting something that hangs broad and loose, attached by only one side. The evolution of the flap courses tortuously over the centuries, illustrating the intense drive for humans to reconstruct deficient anatomy and create form where originally absent. As such, flaps premiered long before the comfort and convenience of anesthesia. In India in 600 BC, Sushruta Samita described operations for nasal reconstruction—a necessity during the pre-Christian era, since amputation of the nose (an organ of "respect and reputation") was common as criminal punishment.
As years passed, many advances regarding flap use arose, including extensive facial flaps and the first cross-leg flap. However, the first muscle flap of recorded history debuted in 1906. Louis Ombredanne of Paris described the use of the pectoralis minor muscle for breast reconstruction following mastectomy. That same year, Tanzini introduced the latissimus dorsi muscle flap for postmastectomy breast reconstruction. As breast surgery became more radical, the human drive toward reconstruction created and stimulated the appearance of the first musculocutaneous flap (ie, muscle in addition to overlying skin and subcutaneous tissue). In 1912, Professor Stefano d'Este introduced this technique. He used the latissimus dorsi muscle and the skin of that same area to reconstruct the chest wall after mastectomy.
Developments in anesthesia, antibiotics, hematology, and wound healing brought about routine use of flaps with good postoperative results. Improvements in surgical technique and advances in microsurgery also resulted in more complex flaps, resulting in composite and compound pedicled and muscle flaps.1,2
Definition
The concepts of a flap and a graft are often confused. In an effort to distinguish between the two terms, their definitions are listed below.
- Flap - A segment of tissue transplanted to a defect while maintaining its own and/or original blood supply
- Graft - A segment of tissue transplanted to a defect when the nutrients for graft survival depend on the recipient site blood supply and not the donor blood supply
Many types of flaps are available, including cutaneous (local), fasciocutaneous, muscle, musculocutaneous, and free flaps. A local skin flap, containing all layers of the skin plus the underlying superficial fascia, restores the integrity of smaller wound defects. The muscle flap uses only muscle for defect coverage. It is used primarily to provide a well-vascularized soft tissue that is relatively resistant to infection, helps wounds heal, and offers a vascularized surface for skin grafts. The muscle flap is commonly used to eradicate infection and to revascularize bone. It is commonly used in several areas, including lower extremity reconstructions (eg, gastrocnemius, soleus, vastus lateralis, latissimus dorsi) and sternal wounds (eg, pectoralis major). When taken with both its nerve and vascular pedicle, muscle flaps can be transferred as functional units for use in extremity and facial reanimation (eg, gracilis, rectus femoris).
When muscle flaps are harvested as composite tissue flaps, they incorporate additional components, such as skin, subcutaneous tissue, fat, and, possibly, bone. Musculocutaneous flaps encompass skin, subcutaneous tissues, and muscle, while myo-osteocutaneous flaps also contain bone. The musculocutaneous flap (skin, subcutaneous tissue, fat, and muscle) can be harvested as a regional pedicled flap that allows for greater rotation distances into a nearby defect than the local cutaneous flap, which requires elevation in close proximity to the defect. Because a skin paddle is provided, the musculocutaneous flap is generally preferred to the muscle flap alone because of its ability to provide a combined replacement of deficient tissue. Chronic skin ulcers, such as pressure sores, that are refractory to conventional local wound therapies are good examples of potential beneficiaries of the musculocutaneous flap. (Click here to complete a Medscape nursing CE activity about pressure ulcers.)
Breast reconstruction and treatment of irradiated wounds are reconstructive cases in which musculocutaneous flaps are commonly and effectively used. Recent reports from the Instituto Nacional de Cancerologia in Mexico City suggest pedicled musculocutaneous flaps are "excellent" options for breast cancer reconstruction in patients with advanced disease, recurrent disease, and radiation complications. This type of flap supplies healthy vascularized tissue to improve healing in otherwise compromised local tissue.
The only potential exception to the myocutaneous preference is in lower extremity defects in which a muscle flap and a skin graft provide a better result than the all-in-one musculocutaneous tissue transfer. Initial studies by Mathes et al showed that muscle flaps likely improved wound healing in infected wounds because of their enhanced bacterial clearance, tissue ingrowth, and vascular perfusion.3,4 As a result, most wounds at high risk for infection have been treated using pedicled or free muscle flaps. However, more recent retrospective studies by Wei et al found no differences between musculocutaneous and fasciocutaneous flaps in wound healing following lower extremity trauma reconstructions.5 Both types of flap healed equally well with comparable infection rates. Unless a large, complex, 3-dimensional defect is present, muscle or myocutaneous flaps may not be necessary.
Fasciocutaneous flaps contain skin, fascia, and subcutaneous tissue. These flaps are appropriate in areas with not as much soft tissue loss. When harvested as a pedicled flap, it should be in close proximity to the defect. Studies boast that fasciocutaneous flaps are easy to mobilize and very reliable. However, many surgeons prefer to use muscle flaps in potentially contaminated wounds because studies have suggested a theoretical advantage in terms of bacterial clearance, flap perfusion, and flap ingrowth into the contaminated area. More recent publications have found no clinical differences in postoperative wound infections between fasciocutaneous and muscle flaps reconstructions, if wounds had been adequately debrided.5,6
Briefly, the free flap (more precisely, the free tissue transfer flap) is an "en bloc" transfer of tissues from a carefully selected donor site, following meticulous flap design and harvest, to a distant (even remote) recipient site. Multiple flaps, chimeric flaps, and composite flaps that contain various tissue types can be transferred on a single vascular pedicle for complex three-dimensional tissue defects.7,2 The nature of this reconstructive technique requires complete division of donor segment blood supply with microvascular reanastomosis of the flap pedicle to the recipient site vasculature. Donor and recipient site vessels normally range between 1-3 mm in diameter. When vessels are smaller than 1 mm in diameter, vascular anastomosis falls into the category of supermicrosurgery.
Both microsurgery and supermicrosurgery are technically demanding procedures and are often an option when local or regional flaps are not feasible, such as in defects resulting from severe traumatic injury. Degloving injuries of the extremities that require subsequent soft tissue coverage is a good example of when microsurgical reconstruction is necessary. The flap algorithm can act as a general guide to aid in the decision of flap choice (see below).
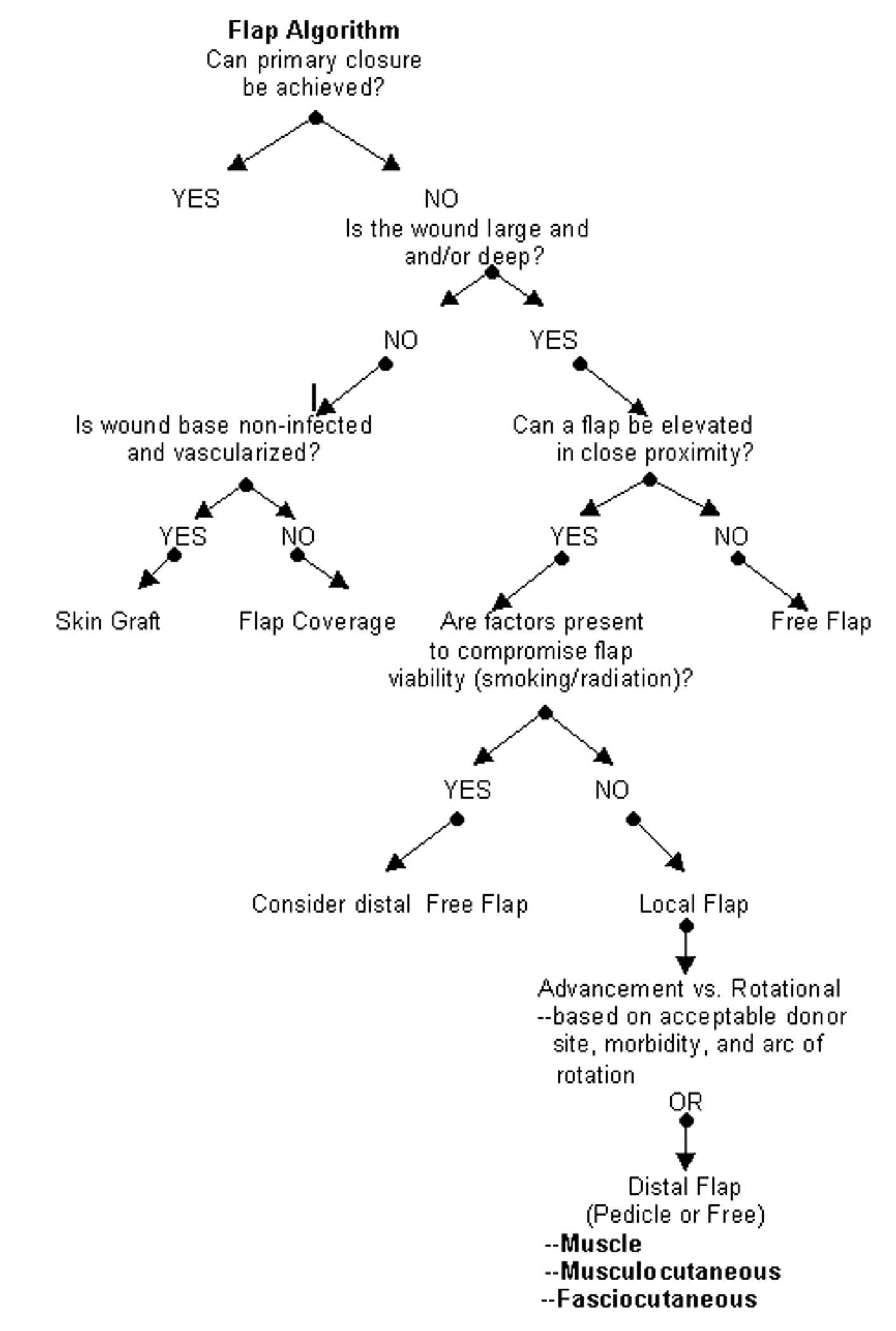
Flap Algorithm
See Image 6 for a flap algorithm to aid in choosing the appropriate flap.
Anatomy
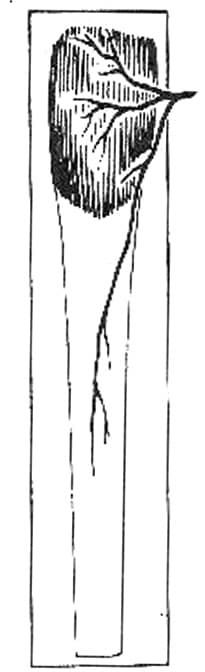
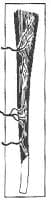
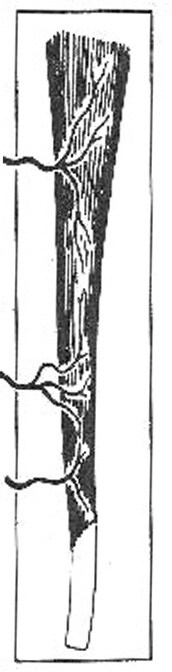
Muscle and musculocutaneous flaps are characterized by their vascular patterns. They have a dominant vascular pedicle that supplies the named muscle and the overlying skin secondary to perforating branches of the dominant vessel. The 5 different vascular patterns of the muscle and musculocutaneous flaps are as follows:
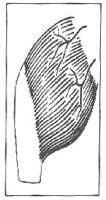
I. Single vascular pedicle (eg, tensor fascia lata; see Image 1)
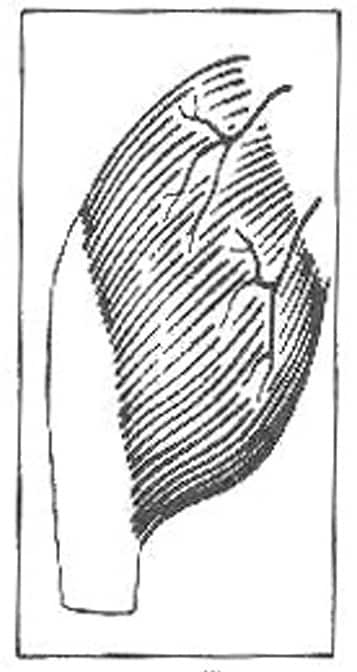
II. Dominant vascular pedicle and minor vascular pedicle (eg, gracilis; see Image 2)
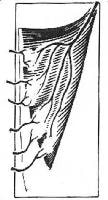
III. Two dominant pedicles (eg, gluteus maximus; see Image 3)
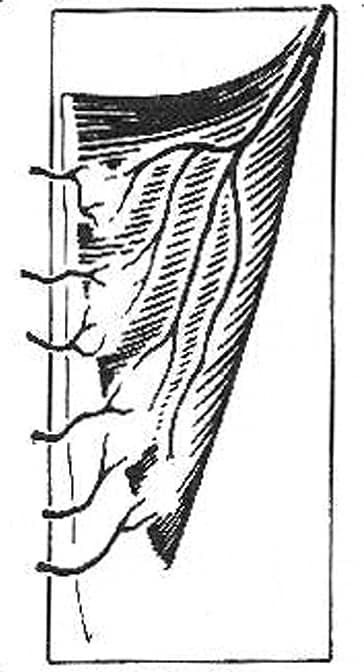
IV. Segmental vascular pedicles (eg, sartorius; see Image 4)
V. Single dominant vascular pedicle and secondary segmental pedicles (eg, latissimus dorsi; see Image 5)
Pathophysiology
Flaps work because of eventual vascular connections between the flap with its nutrient vessels and the recipient site. Approximately 4 weeks are required before homeostasis returns after a tissue transfer. Below are chronologic changes of a flap and the recipient site after elevation and transfer.
- After 10-24 hours - Decreased arterial supply; congestion and edema; dilation of arterioles and capillaries
- After 1-3 days - Increased number and quality of anastomoses between flap and recipient bed; increased number of small vessels in pedicle
- After 3-7 days - Reorientation of vessels along the long axis of the flap; anastomoses created at 1-3 days now functionally significant
- After 1 week - Circulation well established between flap and recipient bed
- After 2 weeks - Continuous maturation of anastomoses
- After 3 weeks - Flap achieves 90% of its final circulation
- After 4 weeks - Vessels at definitive size; few remaining newly formed vessels
Indications
As described briefly in the definition section, the muscle and myocutaneous flaps should be primary considerations when presented with a large and/or deep anatomic defect. This defect can be a surgically planned deficiency such as a postmastectomy wound or the result of trauma or chronic infection. A wound worrisome for infection is a candidate for a muscle or musculocutaneous flap primarily because of its ability to decontaminate. Three important questions relating to a successful flap outcome must be asked prior to flap selection.
- Does the proposed flap provide appropriate coverage for the defect? Stable coverage is essential for flap survival. Simply stated, if the flap bulk is deficient, then the resultant dead space causes subsequent deformity, retraction, seroma formation, and a medium for infection, causing flap demise. Inadequate flap size can worsen the initial problem. Match flap size to donor site.
- How will raising the flap affect the form at both the donor and recipient sites? A general principle for flap selection includes the ability to restore form at the recipient site after tissue transfer and avoidance of donor site deformity after tissue transfer.
- How will raising the flap affect function at the donor site? If the muscle being transferred has a synergistic partner or if it is synergistic in its original function, then raising the flap alters muscle function. Depending on the degree of muscle function alteration, elevating the flap may not be feasible. (This is known as "Rob Peter to pay Paul syndrome.")
Preoperative Preparation
Preoperative assessment of the wound's ability to heal is key. Assess nutritional status, vascular supply, infection, and potential causes for a nonhealing wound. Potential patient comorbidities that affect wound healing include radiation history, nutritional deficits, tobacco use, diabetes, connective tissue diseases, peripheral vascular disease, and obesity. Recipes for jeopardized blood supply and increased infection risk should headline a preoperative checklist.
Researchers at the University of Texas MD Anderson Cancer Center found that obese patients undergoing free transverse rectus abdominis myocutaneous flaps have significantly higher incidence of flap reconstruction complications, such as fat necrosis, flap loss, and donor-site infection.8 Factors that challenge the principles of wound healing should be addressed in the patient prior to flap design.
On occasion, a patient may need an imaging study before undergoing an operation. For example, a preoperative angiogram may be obtained in a patient with a chronic open wound who, at the time of original injury, sustained a crush fracture to the distal lower extremity. If lower extremity pulses are weakened, the arterial study helps map vascular anatomy to the potential donor sites for wound coverage. However, if the planned flap provides accurate coverage, maintains form at both the donor and recipient site, and does not compromise function at the donor site, the workup for a potentially successful muscular flap is complete.
As with all operative intervention, thorough informed consent is a must to obtain. Of specific importance with regard to tissue transfer techniques, the patient must be aware of the risks ranging from pain and scar to deformity and compromise or loss of normal function of the involved tissues. The price for reconstruction is not always solely paid for with currency.
Complications
Errors in judgment, technique, and patient treatment foster complication in muscle and musculocutaneous flap survival. Poor flap design is often a source of flap demise. For example, failure to recognize posttransfer tension and failure to recognize compromised flap blood supply after tissue elevation and transfer can risk flap viability. Both tension on a wound and impaired tissue perfusion represent general flap ischemia. With "reverse planning" (ie, preceding operative intervention with a mock transfer using a piece of fabric and rotating it from the donor site to the recipient site as would be done during the procedure), some technical complications can be intercepted.
Improper flap design (eg, failure to factor nutrition, obesity) leads to seroma formation, hematoma formation, superficial skin necrosis, and wound separation with eventual partial and/or complete flap loss. Seromas generally can be treated with a continuous pressure dressing to promote reabsorption. Conversely, dependent on surgeon preference, the fluid pocket can be actively aspirated with a needle or passively aspirated with a closed suction drainage system.
Intraoperative examination of the flap provides an early opportunity to optimize flap survival. At the time of surgery, the presence of a brisk, bright red blood flow from the distal edge of the flap is the only anatomic test helpful in determining flap viability. Again, the flap must provide adequate coverage under no tension. Keeping the skin edges moist to decrease depth of tissue loss and keeping the flap warm to prevent the vasoconstriction of hypothermia are techniques that can increase the chance of flap survival.
The primary cause of flap demise is not inadequate arterial inflow but rather venous insufficiency (ie, compromise of venous outflow). During venous congestion, necrosis requires several days for definitive demarcation. Areas of concern are those that are bluish in color and that demonstrate a sluggish circulation. Because necrosis is a slow phenomenon, often the elapsed time allows for some revascularization from peripheral tissues at the recipient site. Therefore, instead of complete flap death, tissue is lost centrally, where the revascularization is most challenged. If the warnings of imminent flap death are identified early, then simple maneuvers like taking out sutures/staples at areas of tension and freeing congestion with periodic needle sticks may curb necrosis. Frequently, partial flap salvage is possible. If definitive loss of tissue occurs in an area of the flap, the affected region requires sharp debridement with subsequent local wound care.
Complications such as hematoma formation and complete flap loss may require second-look operative intervention, particularly if persistent bleeding and signs of infection appear, respectively. Complete flap loss is rare, and proper flap design and planning are the keys to successful muscle flap transfer. Korean researchers at the Wonju College of Medicine conclude, based on rodent models, that prostaglandin E1 may increase flap survival. Pending further in vivo success, prostaglandin E1 use could be part of a future musculocutaneous flap protocol.
Multimedia
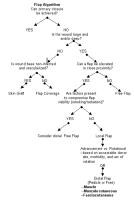 | Media file 6: Flap algorithm. |
Keywords
nasal reconstruction, facial flaps, cross-leg flap, muscle flap, breast reconstruction, latissimus dorsi muscle flap, breast surgery, muscle flap, musculocutaneous flap, nasal repair, nasal surgery, nose reconstruction, nose flap, facial flap
The authors and editors of eMedicine gratefully acknowledge the contributions of previous authors David A Jansen, MD, FACS; Jennifer McGee, MD; Michael Friel, MD, BSE; and R Edward Newsome, MD to the development and writing of this article.



Không có nhận xét nào:
Đăng nhận xét