Introduction
Background
Lupus erythematosus (LE) is an autoimmune disease that can affect the skin, joints, heart, lungs, kidneys, and brain. Drug-induced lupus erythematosus (DILE) is a variant of autoimmune disease that resolves within days to months after withdrawal of the culprit drug in a patient with no underlying immune system dysfunction. Care must be taken to correctly diagnose the symptoms of drug-induced lupus erythematosus and differentiate it from the systemic autoimmune disease, and drug-induced lupus erythematosus should be recognized clinically and serologically for prompt intervention.
Drug-induced lupus erythematosus can arise months to years after exposure to drugs prescribed to treat various medical conditions (eg, antihypertensives, antibiotics, anticonvulsants). The most common drugs that cause drug-induced lupus erythematosus are hydralazine, procainamide, quinidine, isoniazid, diltiazem, and minocycline.1
Although both systemic lupus erythematosus (SLE) and drug-induced lupus erythematosus are autoimmune disorders and can have similar clinical and laboratory features, research suggests different mechanistic pathways. Guidelines for diagnoses and management of SLE have been established.2 Although the pathogenesis of drug-induced lupus erythematosus is not completely understood, a genetic predisposition may play a role, as has been shown with certain drugs metabolized by acetylation, such as procainamide or hydralazine.3 Varying mechanisms leading to the formation of self-recognizing antibodies may explain the differential characteristics of drug effects in persons with drug-induced lupus erythematosus and lupus erythematosus. For example, whereas some drugs can cause drug-induced lupus erythematosus, others may cause a flare of preexisting SLE.
Drugs such as procainamide, chlorpromazine, and quinidine cause the production of antinuclear antibodies against the histone dimer H2A-H2B. Hydralazine forms antinuclear antibodies to H1 and the H3-H4 complex.4 Drugs that cause drug-induced lupus erythematosus usually take months to years before the associated symptoms occur, whereas flares of SLE due to drugs may occur within hours to days.
Drug-induced lupus erythematosus is characterized by improvement upon withdrawal of the offending drug or agent in a patient with a previously normal immune system. No specific criteria establish the diagnosis of drug-induced lupus erythematosus, and excluding underlying autoimmune disease is not a simple process. Obvious clinical or serologic evidence of drug-induced lupus erythematosus is not invariably present, even in rare cases of fatal drug-induced lupus erythematosus. Patients who have serologic and clinical findings that normally indicate SLE might actually have drug-induced lupus erythematosus. The symptoms of both drug-induced flares of SLE and drug-induced lupus erythematosus are temporally related to drug exposure, and SLE and drug-induced lupus erythematosus have similar manifestations. Thus, drug-induced lupus erythematosus is typically diagnosed by a process of elimination to rule out SLE.
Although both lupus erythematosus and drug-induced lupus erythematosus can affect multiple organ systems, including the skin, joints, kidneys, and CNS, complications of drug-induced lupus erythematosus that affect the kidneys and CNS are generally considered rare. In drug-induced lupus erythematosus induced by certain drugs, however, the rate of kidney involvement can be significant. For example, the rate of glomerulonephritis in hydralazine-induced drug-induced lupus erythematosus is 5-10%.
Penicillamine is also more likely to be associated with renal disease. Rare cases of death associated with drug-induced lupus erythematosus have been reported as a direct result of renal complications. Thus, a renal biopsy may be necessary to rule out membranous proliferative and necrotizing glomerulonephritis. Hepatic necrosis is another potential serious complication of drug-induced lupus erythematosus and has been documented in cases of minocycline-induced drug-induced lupus erythematosus.
For proper diagnosis, the following factors should be preliminarily confirmed:
- The patient has one or more clinical symptoms of SLE (eg, arthralgias, lymphadenopathy, rash, fever).
- Antinuclear antibodies are present.
- The patient had no history of SLE prior to using the culprit drug.
- The drug was taken anytime from 3 weeks to 2 years prior to the appearance of symptoms.
- Clinical improvement is rapid when the drug is discontinued, whereas antinuclear antibodies and other serologic markers slowly decrease toward more normal levels.
- Acebutolol
- Amiodarone
- Atenolol5
- Bupropion
- Captopril
- Carbamazepine6
- Chlorpromazine
- Diltiazem7
- Docetaxel
- Ethosuximide
- Gemfibrozil
- Glyburide
- Gold salt
- Griseofulvin
- Hydantoins
- Hydralazine
- Hydroxychloroquine
- Interferons
- Interleukins
- Isoniazid
- Leuprolide acetate
- Lithium
- Lovastatin
- Mephenytoin
- Methyldopa
- Minocycline8
- Nitrofurantoin
- Olanzapine
- Ophthalmic timolol
- Oral contraceptives
- Penicillamine
- Phenytoin
- Practolol
- Procainamide
- Propylthiouracil
- Quinidine
- Reserpine
- Rifampin
- Rifamycin9
- Sertraline10
- Simvastatin
- Sulfasalazine11
- Tetracycline
- Ticlopidine
- Tiotropium bromide inhaler12
- Trimethadione
- Tumor necrosis factor-α (TNF α) (etanercept, infliximab, adalimubab)13
- Valproate
- Voriconazole
- Cimetidine
- Hydralazine
- Hydrochlorothiazide
- Mesantoin
- P -Aminobenzoic acid (PABA)
- Penicillin
- Phenylbutazone
- Sulfonamides
- Terbinafine14
Pathophysiology
Both SLE and drug-induced lupus erythematosus are autoimmune diseases that cause the immune system to manufacture autoantibodies against the patient's own tissues. Which drug characteristics cause the autoantibody formation is unclear, but several theories have been proposed.
One is that the drug serves as a substrate for myeloperoxidase, which is activated in polymorphonuclear neutrophils. This interaction causes the formation of reactive metabolites that directly affect lymphocyte function. A second theory is that with decreased T-cell methylation, an overexpression of lymphocyte function–associated antigen (LFA-1) occurs. T cells with hypomethylated DNA become autoreactive and cause antibody formation. This is the mechanism by which UV light causes flares of lupus. A third theory is that the genetic differences in an individual’s P450 system causes drugs to be metabolized differently, which results in the generation of toxic metabolites that may facilitate autoimmunity.
The medications and other exposures implicated in drug-induced lupus erythematosus and flares of SLE produce autoantibodies more often than systemic autoimmune symptoms. Despite these commonalities, research suggests that drug-induced lupus erythematosus and SLE have separate and distinct mechanistic pathways.
Molecular mimicry between antibodies directed against infectious agents (eg, bacteria, Epstein-Barr virus) and self-antigens has been implicated in SLE. These theories hold that in SLE, the immune system generates autoantibodies to foreign antigens and, in turn, these autoantibodies attack the patient's own tissues.
Autoantibodies in drug-induced lupus erythematosus are thought to be generated by a different mechanism than molecular mimicry. Metabolites of drugs that cause drug-induced lupus erythematosus are subjected to oxidative metabolism by neutrophils, creating reactive metabolites. Virtually all lupus-inducing drugs have been shown to undergo oxidative metabolism, while analogous non–lupus-inducing drugs do not undergo oxidation. The drug metabolite, in turn, is thought to trigger reactions in the thymus that prevent T cells from developing tolerance to the patient's own tissues. In a mouse model, reactive metabolites of procainamide injected into the thymus have been shown to result in lupuslike autoantibodies. Unlike in drug hypersensitivity reactions, this process takes months to years of drug exposure for symptoms to develop.
The production of autoimmune T cells is initiated in the thymus by the capacity of reactive drug metabolites to disrupt central T-cell tolerance. Both mouse model and human studies implicate thymic activity, possibly indicating the persistence of thymic activity into advanced adult life.
Predisposing factors to the development of drug-induced lupus erythematosus include a slow drug-acetylator phenotype and advancing patient age. Slower acetylation may play a role in the greater predisposition for elderly persons to develop drug-induced lupus erythematosus.3 Higher rates of drug-induced lupus erythematosus in elderly persons, however, is also likely due to decreased drug clearance and increased medication usage in these individuals.
Biologics such as interleukins (eg, interleukin 2), interferons (eg, alfa, gamma, beta), and TNF-alpha inhibitors are associated with musculoskeletal symptoms and antibody production suggestive of a lupus-like autoimmune disorder. In one study, approximately 14% of rheumatoid arthritis patients treated with anti–TNF-alpha developed anti-DNA antibodies, while less than 1% developed lupus-like symptoms.
Recognition of DILE in patients receiving anti-TNFα agents can be difficult. Making the diagnosis of DILE is even more challenging because cutaneous reactions with and without evidence of autoimmunity are very common in patients treated with anti-TNFa drugs. Importantly, understand the temporal relationship between the onset of symptoms and the initiation of the medication, which can range from weeks to months. A review by Ramos-Casals et al described 105 patients who developed DILE after starting anti-TNFα therapy, and, in this group, lupuslike symptoms appeared at a mean time of 41 weeks after beginning anti-TNFα therapy.13
As TNFα-targeted therapies are being used for an expanding number of autoimmune diseases, the number of reports of their induction of lupuslike syndromes has been growing. When one considers the large number of patients now treated with these biologic agents, the occurrence of anti-TNFα-induced DILE is relatively rare.15 Most case reports are a result of the use of etanercept or infliximab; adalimumab is less often the inciting agent, which may simply be the result of fewer cumulative patient years of exposure to adalimumab.
Costa et al reported 33 cases of anti-TNFα agents causing DILE. Of these, 21 were due to infliximab, 10 to etanercept, and only 2 cases to adalimumab.16 Of their 33 patients, 19 had only cutaneous manifestations.16 Anti-TNFα agents induce a higher prevalence of antibodies to double-stranded DNA, hypocomplementemia, a higher incidence of both cutaneous and systemic disease, particularly renal involvement, than classic DILE caused by other drugs.
Cutaneous findings in TNFα-associated DILE commonly include photosensitivity and the classic cutaneous findings associated with discoid lupus erythematosus and subacute cutaneous lupus erythematosus. Cutaneous manifestations are more frequently observed in patients receiving etanercept, whereas infliximab causes a higher incidence of serositis. Fever is found in similar incidence in both TNFα inhibitor–induced DILE and DILE caused by other categories of medications. Greater than 50% of laboratory results in anti-TNFα–induced DILE patients show low serum complement levels and anti-dsDNA antibodies, which are usually absent or rare findings in classic DILE.
The use of anti-TNFα agents is also associated with the emergence of other autoantibodies, such as anticardiolipin antibodies. Classic DILE is more often associated with antihistone antibodies. DeBandt et al looked at the only case of thrombosis in a patient with anticardiolipin antibodies and anti-TNFα–induced DILE, although half of the anti-TNFα–related DILE patients studied (12) had anticardiolipin antibodies.
The mechanism by which anti-TNFα therapy induces DILE is not understood. One hypothesis is that the binding of the anti-TNFα drug to the cell surface TNFα induces cell apoptosis, which causes the release of antinucleosomal autoantigens and the induction of anti-dsDNA antibodies.16 A second hypothesis is that the suppression of T-helper type 1 response from the anti-TNFα therapy would generate an exuberant T-helper 2 response, leading to an overproduction of autoantibodies.17 A third suggestion to the pathogenesis of DILE from these immunosuppressive agents is that patients on these medications may experience more bacterial infections, which are powerful stimulants that boost polyclonal B-lymphocyte activation and autoantibody production.18
A baseline evaluation, including serology to rule out lupus erythematosus, should be considered in patients prior to the inception of TNFα therapy.16
Comparison of Findings Between Drug-induced Lupus Erythematosus and Systemic Lupus Erythematosus
Open table in new window
Table
| Findings | SLE | Drug-induced Lupus Erythematosus |
|---|---|---|
| Clinical | Average age of onset of 20-30 y Affects blacks more than whites Female-to-male ratio of 9:1 | Average age of onset of 50-70 y Affects whites more than blacks Female-to-male ratio of 1:1 |
| Laboratory | Antihistone antibodies in 50% Anti-dsDNA present in 80% C3/C4 levels decrease Cutaneous findings in >75% Raynaud phenomenon in 50% Antinuclear antibodies in >95% | Antihistone antibodies in >95% Anti-ssDNA present Anti-dsDNA rare C3/C4 levels normal Cutaneous findings in ~25% Raynaud phenomenon in 25% Antinuclear antibodies in >95% |
| Immunofluorescence Histopathology | Direct immunofluorescence reveals granular deposition of immunoglobulin G at dermoepidermal junction Lymphohistiocytic interface dermatitis Apoptosis basal vacuolization | Same as SLE Same as SLE |
| Findings | SLE | Drug-induced Lupus Erythematosus |
|---|---|---|
| Clinical | Average age of onset of 20-30 y Affects blacks more than whites Female-to-male ratio of 9:1 | Average age of onset of 50-70 y Affects whites more than blacks Female-to-male ratio of 1:1 |
| Laboratory | Antihistone antibodies in 50% Anti-dsDNA present in 80% C3/C4 levels decrease Cutaneous findings in >75% Raynaud phenomenon in 50% Antinuclear antibodies in >95% | Antihistone antibodies in >95% Anti-ssDNA present Anti-dsDNA rare C3/C4 levels normal Cutaneous findings in ~25% Raynaud phenomenon in 25% Antinuclear antibodies in >95% |
| Immunofluorescence Histopathology | Direct immunofluorescence reveals granular deposition of immunoglobulin G at dermoepidermal junction Lymphohistiocytic interface dermatitis Apoptosis basal vacuolization | Same as SLE Same as SLE |
Frequency
United States
As many as 10% of the approximately 500,000 cases of lupus erythematosus may be drug-induced lupus erythematosus.
Mortality/Morbidity
Death from drug-induced lupus erythematosus is extremely rare and may result from renal involvement. In diagnosing drug-induced lupus erythematosus, first excluding the possibility of renal idiopathic lupus rather than drug-induced lupus erythematosus is extremely crucial.
Race
More whites than blacks develop drug-induced lupus erythematosus; more blacks than whites present with SLE.
Sex
In drug-induced lupus erythematosus, no significant statistical difference is apparent in the prevalence for males versus females. In contrast, SLE affects women with considerably higher frequency than men (female-to-male ratio of 9:1).
Age
Patients with drug-induced lupus erythematosus tend to be older (50-70 y) than those with SLE (average age 29 y at diagnosis). Elderly persons generally are more susceptible to drug-induced lupus erythematosus.
Clinical
History
- Most patients with drug-induced lupus erythematosus (DILE) have one or more clinical symptoms of systemic lupus erythematous (SLE), such as arthralgias, lymphadenopathy, rash, and fever, and have had no prior history of autoimmune disease. If a rash is present, it often manifests as a polycyclic, scaling, erythematous rash in sun-exposed areas.
- Approximately 50% of patients have constitutional symptoms of fever, weight loss, and fatigue.
- As many as 90% of patients with drug-induced lupus erythematosus have severe but usually noninflammatory joint pain; however, synovitis may be present.
- Arthralgia is often the only clinical manifestation of drug-induced lupus erythematosus.
- As many as 50% of patients with drug-induced lupus erythematosus experience muscle pain (myalgia).
- The drug was taken anytime from 3 weeks to 2 years prior to the appearance of symptoms. Importantly, note that drug-associated exacerbations of SLE and typical drug hypersensitivities can also be temporally related to drug exposure.
- Clinical improvement is usually rapid when the drug is discontinued, while antinuclear antibodies and other serologic markers slowly decrease toward more normal levels.
- Generally, the absence of CNS and renal involvement is more suggestive of drug-induced lupus erythematosus than SLE. High rates (ie, 5-10%) of glomerulonephritis; however, occur in hydralazine-induced drug-induced lupus erythematosus, and rare cases of death from renal involvement in drug-induced lupus erythematosus have been reported.
Physical
- Extracutaneous physical findings can include the following:
- Splenomegaly
- Hepatomegaly
- Inflammation of the serous membranes that surround the lungs and pleural cavity walls (pleurisy)
- Fever
- Inflammation of the fibroserous membranes that cover the heart and the initial part of the great vessels (ie, pericarditis)
- Cerebritis (rarely)
- Nephritis (rarely)
- Skin findings are apparent in approximately 25% of all patients diagnosed with drug-induced lupus erythematosus. Importantly, note that certain manifestations typical in persons with SLE are not usually observed in persons with drug-induced lupus erythematosus. Patients with drug-induced lupus erythematosus (unlike patients with SLE) typically do not have the following:
- Mucosal ulcers
- Hair loss (alopecia)
- Circular (discoid) plaques
- Photosensitivity (with the exception of thiazide-induced subacute lupuslike syndrome)
- Compared with patients who have SLE, patients with drug-induced lupus erythematosus present with a higher prevalence of the following:
- Purpura
- Erythema nodosum (painful nodules, usually on the extremities)
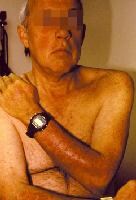
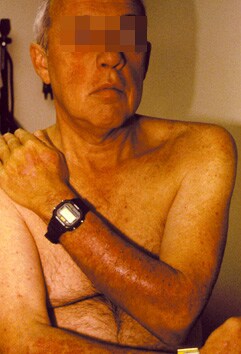
- Erythematous papules (typically on sun-exposed areas, as in the image below)
- Lymphadenopathy or Raynaud phenomenon is present in approximately 35-50% of patients with SLE but in less than 25% of those with drug-induced lupus erythematosus.
- More than 75% of patients with SLE also have cutaneous findings, versus an average of less than 25% in patients with drug-induced lupus erythematosus. However, in both SLE and drug-induced lupus erythematosus, approximately 75% of patients have arthritis or arthralgia.
- Patients with drug-induced lupus erythematosus also occasionally exhibit skin findings that are analogous to those manifested in patients with subacute cutaneous lupus erythematosus, such as erythematous annular or scaly plaques.
Causes
Drug-induced lupus erythematosus may be induced by medications or caused by other compounds in the environment. The most common drugs that cause drug-induced lupus erythematosus are hydralazine (rate roughly 20%), procainamide (rate roughly 20%, 5-8% if taken for 1 y), quinidine, and minocycline.
- Several broad drug categories have been linked to drug-induced lupus erythematosus, including the following:
- Antiarrhythmics - Procainamide and quinidine
- Antibiotics - Minocycline and isoniazid
- Antifungals - Griseofulvin and voriconazole
- Anticonvulsants - Valproate, ethosuximide, carbamazepine, and hydantoins
- Hormonal therapy - Leuprolide acetate
- Antihypertensives - Hydralazine, methyldopa, and captopril
- Anti-inflammatories - Penicillamine and sulfasalazine
- Antipsychotics - Chlorpromazine
- Cholesterol-lowering agents - Lovastatin, simvastatin, and gemfibrozil
- Biologics - Interleukins (eg, interleukin 2), interferons (eg, alfa, beta, gamma), and TNFα
- Inhalers - Tiotropium bromide inhaler
- Other drug categories - Ophthalmic timolol
- A genetic predisposition may play a role. Hydralazine-induced drug-induced lupus erythematosus has been observed with increased frequency in association with human leukocyte antigen (HLA)-DR4.
- Intrinsic genetic susceptibility may help explain why some patients experience drug-induced lupus erythematosus as a reaction to drug therapies, while others do not. For example, the rate of acetylation is genetically predetermined. In the United States, the population is almost evenly divided between those who are fast acetylators and those who are slow acetylators. Those with slow acetylation rates have a higher prevalence of drug-induced lupus erythematosus than those with faster acetylation rates. In contrast, SLE affects individuals with slow and fast acetylation rates approximately equally.
- Other causes may induce drug-induced lupus erythematosus in certain individuals for no apparent reason, such as sensitivity to the following:
- Insecticide compounds
- Certain metallic compounds
- Eosin (a fluorescent acid dye found in some lipsticks)
I was diagnose with genital warts since 2012 i have be taking lot treatment and all i got is outbreak. in 2015 I gave up the treatment because I can't continues wasting time and money on treatment at the end it will not cure me. about 6 weeks ago i did natural research online I had So many people talking good about natural remedy, after the research i was recommended to Dr onokun, And I wrote to him through his email and told him my problem after some conversations with him he gave me natural treatment after 1 week Dr onokun treated me i got cured permanently. and i went to see my doc he confirmed that the diseases has gone out from my body. every patients should know there is 100% natural hpv cure. contact Dr onokun his email address: dronokunherbalcure@gmail.com
Trả lờiXóa